RT-PCR Kits
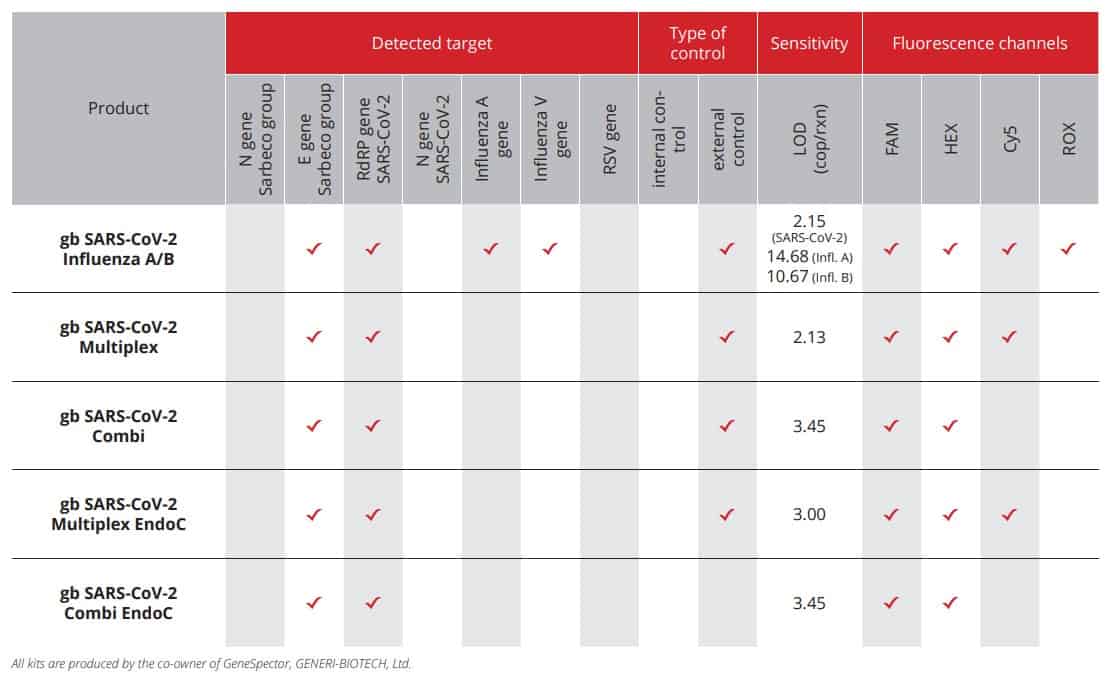
GeneSpector provides a wide range of CE IVD kits for detection of SARS-CoV-2 virus (Wuhan coronavirus 2019) based on one-step RT-qPCR using fl uorescently labelled probes. The detection method is based on officially recommended WHO designs for SARS-CoV-2 virus detection and the assessments originate from protocols published by Charité Laboratories (Berlin), Institut Pasteur (Paris) and CDC Laboratories (Atlanta). Our proprietary GEMINI™ probe technology used guarantees the high detection sensitivity.
We offer a fast (65 minutes) protocol under CE-IVD that is suitable for massive testing.
SARS-CoV-2 Influenza A/B
Detection method: one-step real-time PCR
SARS-CoV-2 Influenza A/B CE IVD kit is based on one-step RT-qPCR using fluorescently labelled probes enabling a multiplex detection of viral genes of SARS-CoV-2, Influenza A and Influenza B. The detection method is based on officially recommended WHO and Institut Pasteur designs for detection of SARS-CoV-2 and on usage of our proprietary GEMINI™ probe technology.
Ordering information
3233-100 SARS-CoV-2 Influenza A/B – 100 pcs
3233-500 SARS-CoV-2 Influenza A/B – 500 pcs
SARS-CoV-2 Multiplex
Detection method: one-step real-time PCR
Multiplex CE IVD kit for detection of SARS-CoV-2 virus is based on one-step RT-qPCR using fluorescently labelled probes (FAM and HEX channels) and external positive control (Cy5 channel). The detection method is based on officially recommended WHO and Institut Pasteur designs for detection of SARS-Cov-2 and usage of our proprietary GEMINI™ probe technology.
Ordering information
3231-050 SARS-CoV-2 Multiplex – 50 pcs
3231-500 SARS-CoV-2 Multiplex – 500 pcs
SARS-CoV-2 Combi
Detection method: one-step real-time PCR
Combi CE IVD kit for detection of SARS-CoV-2 virus is based on one-step RT-qPCR using fluorescently labelled probes which allow the detection of viral genes E and RdRP in FAM channel and external positive control in HEX channel. The detection method is based on officially recommended WHO and Institute Pasteur designs for detection of SARS-Cov-2 and usage of our proprietary GEMINI™ probe technology.
Ordering information
3232-100 SARS-CoV-2 Combi – 100 pcs
3232-500 SARS-CoV-2 Combi – 500 pcs
SARS-CoV-2 Multiplex EndoC
Detection method: one-step real-time PCR
Multiplex CE IVD kit for detection of SARS-CoV-2 virus is based on one-step RT-qPCR using fluorescently labelled probes (FAM and HEX channels) and human endogenous control control (Cy5 channel). The detection method is based on officially recommended WHO and Institut Pasteur designs for detection of SARS-Cov-2 and usage of our proprietary GEMINI™ probe technology.
Ordering information
3234-100 SARS-CoV-2 Multiplex EndoC – 100 pcs
3234-500 SARS-CoV-2 Multiplex EndoC – 500 pcs
Partnering
We have designed our viRNAtrap™ Collection Tubes, viRNAtrap™ Extraction Kit, PCR kit for rapid COVID-19 detection to fit the needs of any laboratory. Our system provides a robust and safe solution for high-throughput COVID-19 diagnostics. therefore, We are therefore open to expand our current customer base by partnering with global players and/or channel partners from all geographies. Get in touch with us.
We are improving molecular diagnostics
PetrskA 1180/3 Praha 1
Czech Republic
info@genespector.com
